electromagnetic bonds. These bonds are in continuous motion, vibrationg
and rotating about thie center of gravity. The atoms of each molecule
vibrate at a definite frequency with respect to each other, the frequency
being specific for that molecular structue and for no other. In IR analysis,
infrared radiation is used to study these vibrations. The short rane ordering
of nearest neighbor atoms is often most clearly shown by infrared analysis.
IR has been used to study both organic and inorganic compounds. It is
more well suited for organic compounds that does not have long range
ordering. Materials that are succesfully analyzed include polymers, organic
compounds and hydrocarbons.
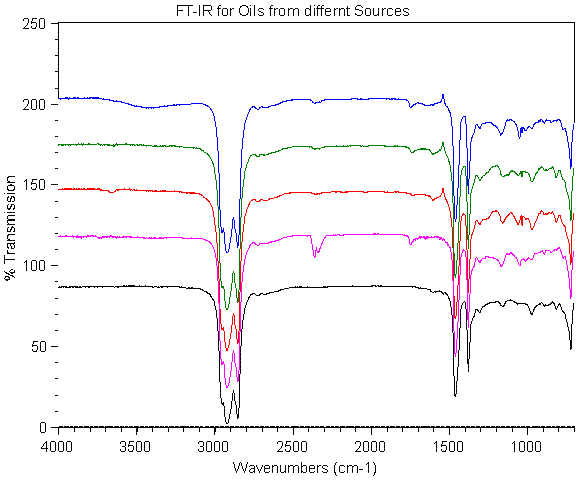
An IR spectrum for an oil sample is shown in the attached figure. In the
figure, the band seen around 2700-2800 cm-1 is from C-H stretching in the
hydrocarbons.
| |
| |
| |
| |
| |
| |
| |
| |